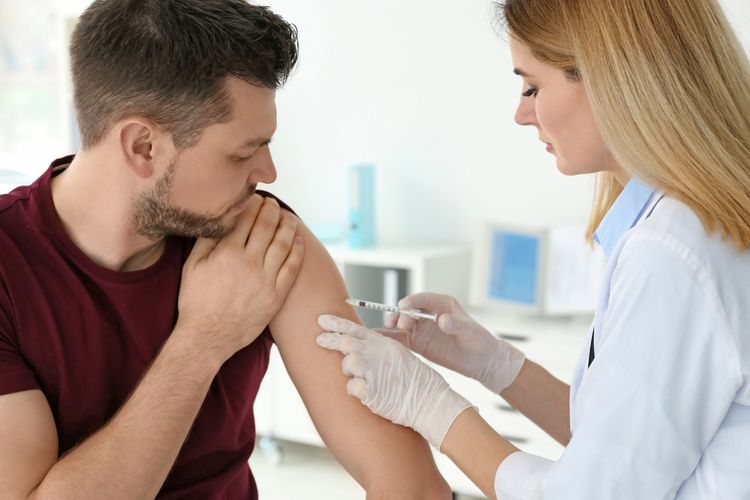
Johnson & Johnson’s Covid-19 Vaccine Trial Paused Over Unexplained Illness
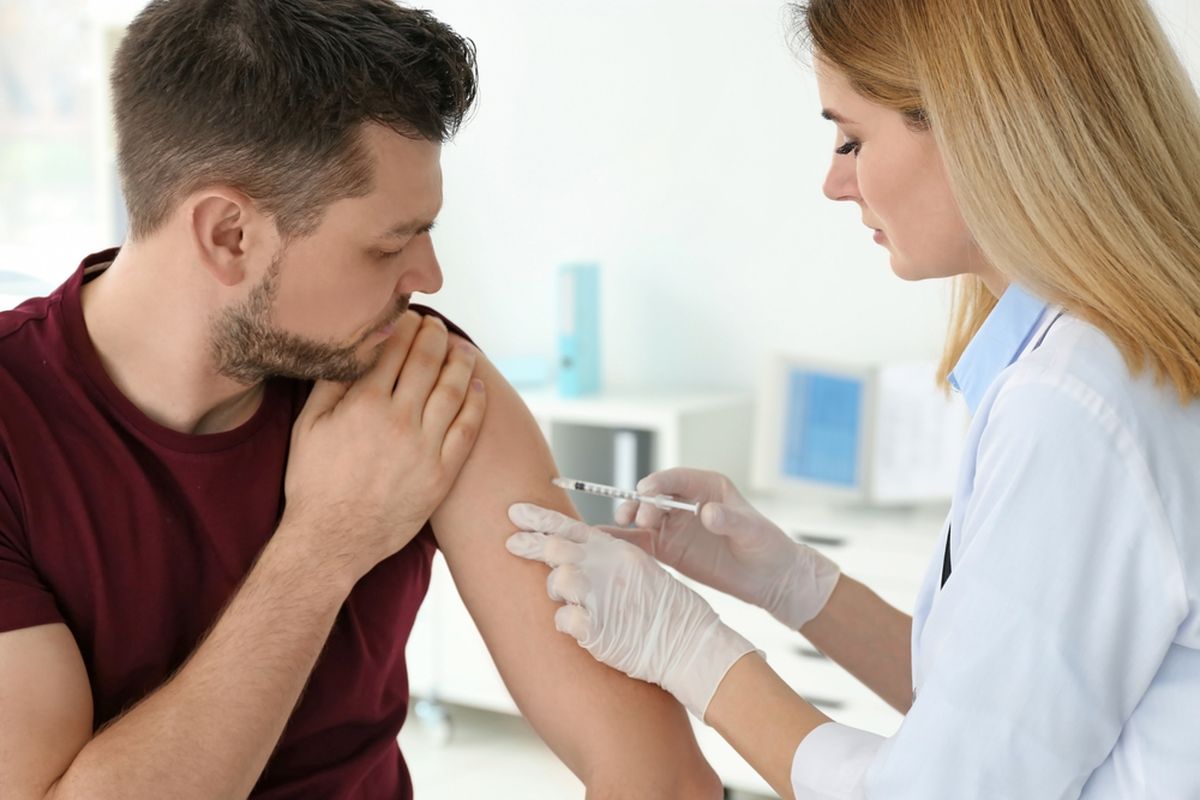
NEW BRUNSWICK, KOMPAS.com - A Covid-19 vaccine trial by Johnson & Johnson has been paused after a study participant incurred an “unexplained illness”.
The late-stage study of the Covid-19 vaccine gives the company time to investigate whether the participant’s illness is related to the shot.
Johnson & Johnson said in a statement Monday evening that illnesses, accidents and other so-called adverse events “are an expected part of any clinical study, especially large studies”.
Nonetheless, its physicians and a safety monitoring panel would try to determine what might have caused the illness.
Read also: AstraZeneca Covid-19 Vaccine Under US Investigation Over Safety
Johnson & Johnson’s abrupt halt of its Covid-19 vaccine trial is at least the second such hold to occur among several vaccines that have reached large-scale final tests in the US.
The company declined to reveal any more details about the illness, citing the participant’s privacy.
Temporary stoppages of large medical studies are relatively common. Few are made public in typical drug trials, but the work to make a coronavirus vaccine has raised the stakes on these kinds of complications.
Companies are required to investigate any serious or unexpected reaction that occurs during drug testing.
Given that such tests are done on tens of thousands of people, some medical problems are a coincidence.
Read also: Indonesia Hopes to Begin Administering Covid-19 Vaccines by November 2020
In fact, one of the first steps the company said it will take is to determine if the person received the vaccine or a placebo. The halt was first reported by the health news site STAT.
Final-stage testing of a vaccine made by AstraZeneca and Oxford University remains on hold in the US as officials examine whether an illness in its trial poses a safety risk.
That trial was stopped when a woman developed severe neurological symptoms consistent with transverse myelitis, a rare inflammation of the spinal cord, the company has said. That company's testing has restarted elsewhere.
Johnson & Johnson was aiming to enroll 60,000 volunteers to prove if its single-dose approach is safe and protects against the coronavirus. Other vaccine candidates in the US require two shots.
(Writer & Editor: The Associated Press)
Source: https://apnews.com/article/virus-outbreak-archive-0843b8b1b0b67e4109d3eb7ff3698de4
Simak breaking news dan berita pilihan kami langsung di ponselmu. Pilih saluran andalanmu akses berita Kompas.com WhatsApp Channel : https://www.whatsapp.com/channel/0029VaFPbedBPzjZrk13HO3D. Pastikan kamu sudah install aplikasi WhatsApp ya.