Indonesia Reissues Controversial AstraZeneca Vaccine Batch

KOMPAS.com – Indonesia has reissued AstraZeneca’s CTMAV 547 Covid-19 vaccine batch, less than two weeks after it was withdrawn from circulation on Sunday, May 16 following the death of a number of the vaccine’s recipients.
The Food and Drug Agency or BPOM cleared the batch for use on May 29, after it put the vaccine through a series of sterilization and abnormal toxicity tests at its National Center for the Development and Testing of Medicines and Foods [PPOMN].
“The tests are part of BPOM investigations to determine if there are any connections between the vaccine’s and side effects such as blood clots, fevers or headaches,” said BPOM in a press release.
“We try to determine if the distribution and storing process will affect the quality of the vaccine. The CTMAV 547 vaccine batch are shown to have normal toxicity and sterility levels, and there are no connections between the vaccine and any post immunization incidents.”
Also read: Indonesia AstraZeneca Vaccine Recipient in Bali Dies
BPOM’s findings determined that the CTMAV 547 vaccine batch, which came to Indonesia on April 26 via the Covax Facility and World Health Organization [WHO], can be used to immunize the Indonesian public.

The National Commission for Post Immunization Incidents or Komnas KIPI also ruled that Covid-19 vaccines from AstraZeneca have not killed anyone in Indonesia.
“Those who died after they received a Covid-19 vaccination did not die of the vaccine, but from other [health] factors,” said the Commission. “The AstraZeneca vaccine should continue to be distributed because it helps combat Covid-19.”
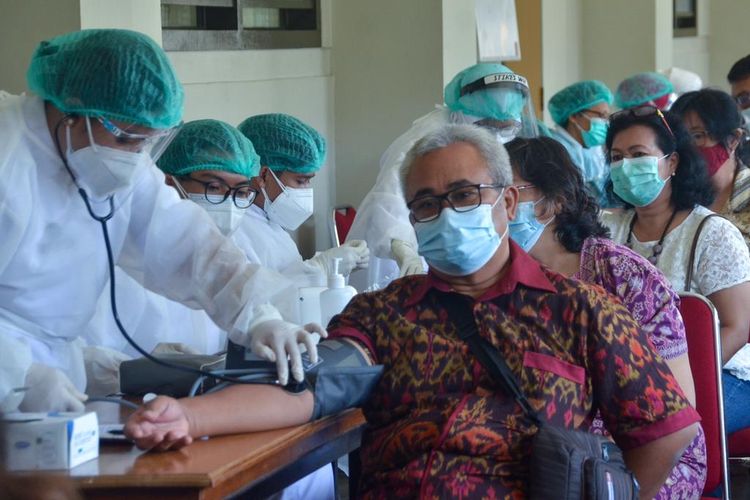 A mass vaccination at the Yogyakarta campus of Atma Jaya University, Thursday, (15/4/2021).
A mass vaccination at the Yogyakarta campus of Atma Jaya University, Thursday, (15/4/2021). The World Health Organization [WHO] and EMA or European Medical Association review of the AstraZeneca vaccine also dispelled concerns about the vaccine causing blood clots.
“Blood clots occurred in in 222 cases out of 34 million doses [of the AstraZeneca vaccine] or 0.00065 percent,” the report said. “On the other hand, blood clots from Covid-19 occurred in 165 thousand cases out of every one million people [who were infected], or 16.5 percent.”
Indonesian Ministry of Health spokesperson for Covid-19 vaccines Siti Nadia Tarmizi hailed the decision to put the CTMAV 547 batch back in circulation.
Also read: Indonesia Halts Use of an AstraZeneca Covid-19 Vaccine Batch After Death of Vaccine Recipient
“The tests [run by BPOM] showed that the AstraZeneca CTMAV 547 vaccine batch is cleared for use again,” she said, as quoted in the Ministry of Health’s website sehatnegeriku.kemkes.go.id.
Indonesia received the CTMAV 547 batch on April 26, 2021 through the Covax Program/WHO initiative. The batch, which numbered 448,480 doses, is part of the 3,852,000 that Indonesia is set to receive in stages.
The batch has been used to vaccinate military personnel as well as members of the public, most notably in the provinces of Jakarta and North Sulawesi.
(Writer: Jawahir Gustav Rizal | Editor : Rizal Setyo Nugroho)
Sources:
Simak breaking news dan berita pilihan kami langsung di ponselmu. Pilih saluran andalanmu akses berita Kompas.com WhatsApp Channel : https://www.whatsapp.com/channel/0029VaFPbedBPzjZrk13HO3D. Pastikan kamu sudah install aplikasi WhatsApp ya.- BPOM
- World Health Organization
- WHO
- COVAX
- AstraZeneca
- Indonesia Covid-19 vaccine
- ema
- Indonesian Ministry of Health
- Komnas KIPI
- siti nadia tarmizi
- AstraZeneca Covid Vaccine Doses
- COVAX program
- astrazeneca vaccine indonesia
- AstraZeneca batch CTMAV547
- indonesia suspends astrazeneca vaccine
- indonesia astrazeneca vaccine
- indonesia astrazeneca death
- indonesia astrazeneca approval
- astrazeneca covid vaccine indonesia
- astrazeneca suspended why
- astrazeneca suspended in indonesia
- Indonesia Covid-19
- astrazeneca vaccine in indonesia