Indonesia Issues Emergency Use Authorization to AstraZeneca’s Covid-19 Vaccine

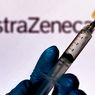
JAKARTA, KOMPAS.com – Indonesia’s Food and Drug Monitoring Agency (BPOM) has issued emergency use authorization to AstraZeneca’s Covid-19 vaccine produced by UK pharmaceutical firm.
“BPOM granted emergency use authorization [EUA] to AstraZeneca’s Covid-19 vaccine on February 22 under the EUA number 2158100143A1,” BPOM head Penny Lukito told a press conference which was held virtually on Tuesday, March 9.
Penny said that the application for the emergency use of the Covid-19 vaccine was done multilaterally and registered directly by AstraZeneca Indonesia.
Also read: Indonesia Receives 1 Million Doses of Astrazeneca’s Covid-19 Vaccine
"It was registered through the bilateral production line of AstraZeneca in Europe and Siam Bioscience in Thailand," she said.
She said before granting the emergency use permit, BPOM had evaluated with the National Committee for Drug Evaluation and other relevant parties.
She further added that they had estimated the efficacy of the vaccine was 62.1 percent.
Also read: Indonesian Government Urged to Step Up Covid-19 Vaccine Drive
Indonesia received the first shipment of more than a million doses of AstraZeneca’s Covid-19 vaccine on Monday, March 8.
Foreign Minister Retno Marsudi said it was the first batch of vaccines that Indonesia received through the multilateral channel. During the first phase, which runs until May, Indonesia will receive a total of 11,748,000 ready-to-use vaccines.
"Insya Allah [God willing], next batches will follow suit,” she said.
(Writer: Haryanti Puspa Sari | Editor: Kristian Erdianto)
Simak breaking news dan berita pilihan kami langsung di ponselmu. Pilih saluran andalanmu akses berita Kompas.com WhatsApp Channel : https://www.whatsapp.com/channel/0029VaFPbedBPzjZrk13HO3D. Pastikan kamu sudah install aplikasi WhatsApp ya.