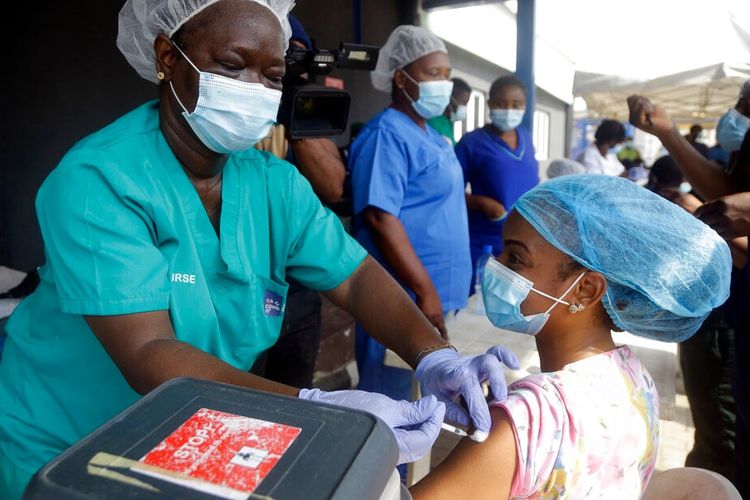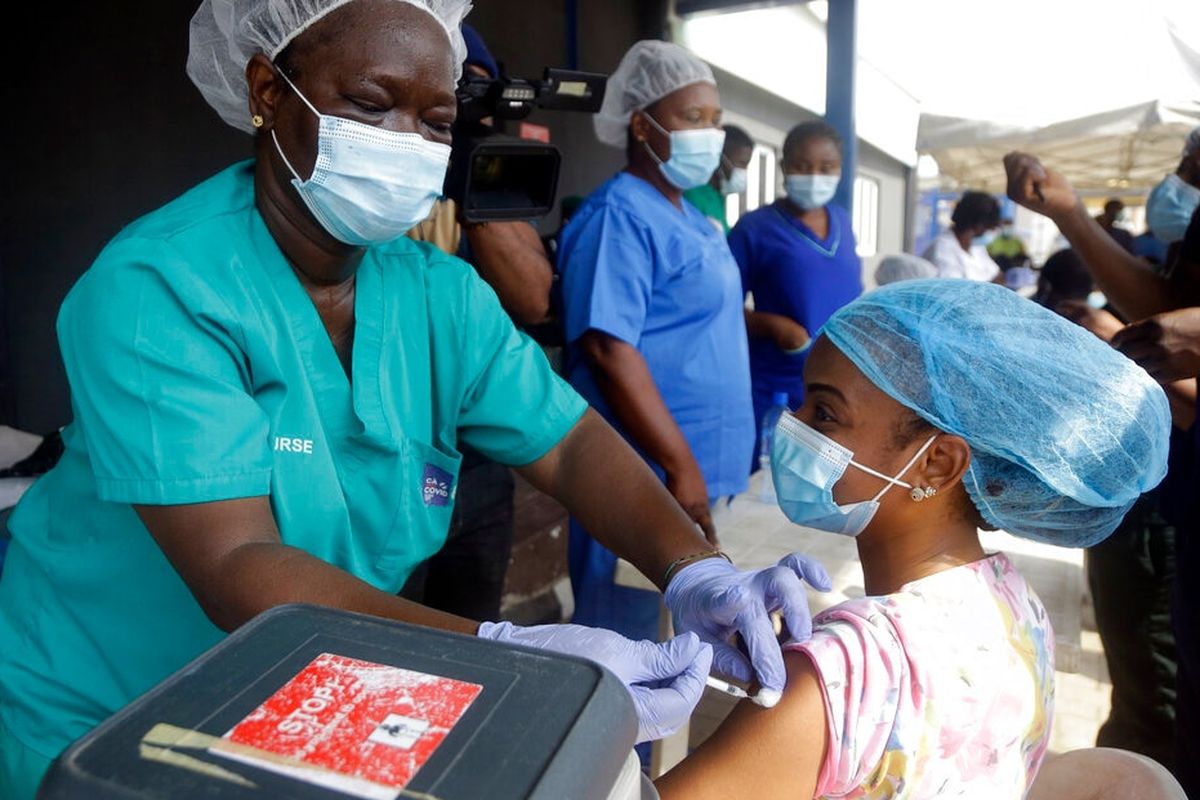
KOMPAS.com - The World Health Organization (WHO) on Friday approved Johnson & Johnson’s COVID-19 vaccine for emergency use, while also firmly endorsing AstraZeneca’s COVID-19 vaccine as some countries continued to suspend its use.
WHO spokeswoman Margaret Harris described AstraZeneca’s vaccine at a virtual briefing hosted in Geneva as “excellent” and said that “we should continue” to use the vaccine.
The WHO endorsement came as Thailand followed an increasing number of European countries in suspending AstraZeneca’s use because of periodic blood clots among recipients.
Bulgaria and the Democratic Republic of the Congo also suspended AstraZeneca use Friday. However, AstraZeneca said in response to the claims that there was “no evidence of an increased risk."
 Health workers and fireman sit under observation after receiving doses of the Oxford-AstraZeneca coronavirus vaccine, in Guatemala City, Guatemala, March 10, 2021
Health workers and fireman sit under observation after receiving doses of the Oxford-AstraZeneca coronavirus vaccine, in Guatemala City, Guatemala, March 10, 2021The European Medicines Agency, a European Union body that supervises medical products, said in a statement that the AstraZeneca vaccine’s “benefits continue to outweigh its risks and the vaccine can continue to be administered while investigation of cases of thromboembolic events is ongoing.”
Thromboembolic events occur when a blood clot breaks loose and travels through the body, causing harm.
The WHO had already approved the AstraZeneca vaccine for global emergency use and on Friday broadened global access to inoculations by approving Johnson & Johnson’s vaccine, the first to be administered in a single injection instead of two. The WHO also previously backed the vaccine by Pfizer-BioNTech, making for a total of three vaccine approvals.
“Every new, safe and effective tool against COVID-19 is another step closer to controlling the pandemic,” WHO Director-General Tedros Adhanom Ghebreyesus said in a statement.
In another development Friday, Brazil reported 2,216 COVID-19 deaths in the last 24 hours, the third day in a row that fatalities in the country exceeded 2,000. The stretch of deaths is the worst in Brazil since the pandemic began last year.
Brazil also reported 85,663 new cases of the coronavirus infection Friday, the second-highest number for a day.
Tedros described the situation in Brazil as “deeply concerning” and said that “unless serious measures are taken, the upward trend now flooding the health system and becoming more than its capacity will result in more deaths.”
 President Joe Biden speaks during a virtual meeting with Indian Prime Minister Narendra Modi, Australian Prime Minister Scott Morrison and Japanese Prime Minister Yoshihide Suga, from the State Dining Room of the White House, March 12, 2021.
President Joe Biden speaks during a virtual meeting with Indian Prime Minister Narendra Modi, Australian Prime Minister Scott Morrison and Japanese Prime Minister Yoshihide Suga, from the State Dining Room of the White House, March 12, 2021.Also Friday, the United States, Australia, India and Japan agreed to a partnership to make 1 billion vaccines available across Asia by the end of 2022, India’s foreign secretary said at a news conference in New Dehli after a virtual meeting with U.S. President Joe Biden and the leaders of the other countries.
At the virtual summit held by the four countries, the Quadrilateral Security Dialogue, commonly known as the “Quad,” the countries agreed to finance, manufacture and distribute the vaccine across Asia.
The initiative is designed to attack the global vaccine shortage and counter China’s growing diplomatic campaign to distribute vaccines in Southeast Asia and globally.