Indonesia to Follow Global Standards for Safe Covid-19 Vaccine Distribution
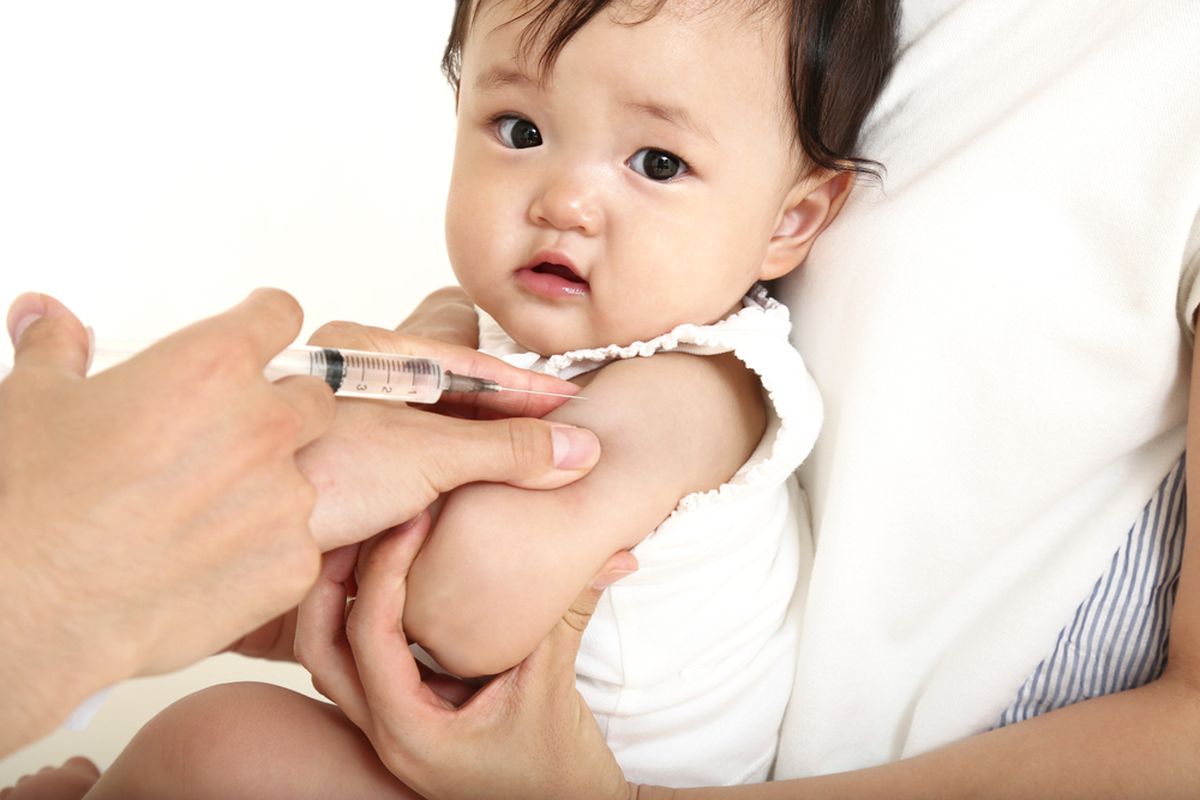
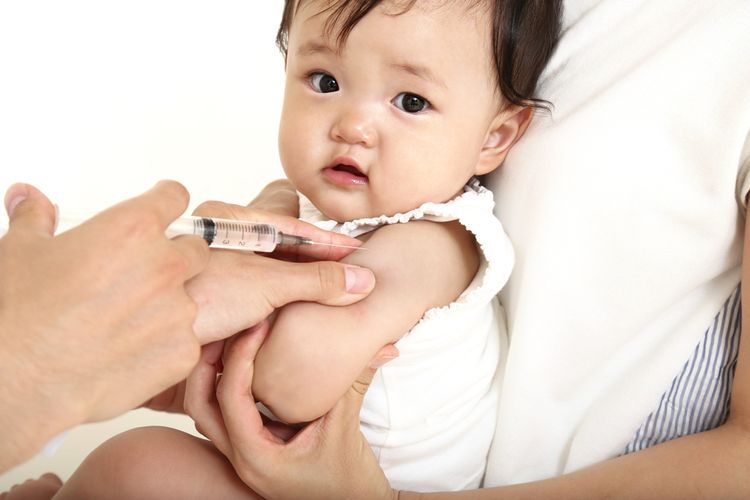
JAKARTA, KOMPAS.com – Sri Mulyani reassured the public that Indonesia’s Covid-19 vaccine will be distributed safely and effectively in keeping with President Joko ‘Jokowi’ Widodo’s instructions.
Indonesia’s Finance Minister said the safe distribution of a Covid-19 vaccine in Indonesia means that all scientific aspects must meet the recommended standards or fulfill the internationally adopted standards.
“To ensure its safety, the vaccine must pass proper clinical trials to give peace of mind to the public.”
The success and result of vaccine clinical trials as well as international safety standards determined by reputable bodies such as the WHO and the Technical Advisory Group on Immunization (ITAGI) are essential to rolling out Indonesia’s Covid-19 vaccination plans.
Read also: Indonesian Foreign Minister Endorses Bio Farma Covid-19 Vaccine
Sri Mulyani emphasized that Indonesia will follow international standards and that the country will not revert to any other standards.
The decision to follow international standards is to show that the Indonesian government has not accelerated Covid-19 vaccine distribution nor conducted its own mechanism of a proper coronavirus vaccine.
“By adopting the WHO and ITAGI’s standards, Indonesia will be able to start its coronavirus vaccination program even if the vaccines originate from different brands,” the Finance Minister said.
Due to the substantial amount of coronavirus vaccines needed in Indonesia — 267 million doses to be exact — the government has used this period to create a detailed plan.
Read also: Priority Recipients of Covid-19 Vaccine Will Be Medical Workers, Working-Age Indonesians
Indonesia’s Finance Minister shared that one of the plans is to create a simulation of a vaccination roll-out in Bogor, Bali, and Ambon, as advised by the country’s Ministry of Health.
The simulations are to give the government an idea of how to execute a Covid-19 vaccination program once the vaccine is ready.
Indonesia’s Ministry of Health is also conducting an evaluation of healthcare providers that either have or must equip themselves with cold storage facilities to avoid damaging the vaccines in accordance with WHO standards.
“According to the health ministry, 90 percent of all healthcare providers have met the WHO’s standards on cold storage facilities. We still need to provide more cold storage facilities to ensure that all future vaccines will be safely stored.”
Read also: Covax Initiative to Supply Covid-19 Vaccine to World’s Poor Faces Trouble