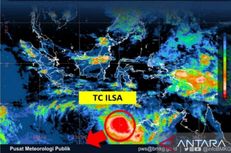
Western Coronavirus Vaccine Producers Pledge to Uphold Testing Rigor

Participants and those working on the trial must not know which group they belong to, according to the pledge.
BioNTech's Sahin added there must be statistical certainty of 95 percent, in some cases higher, that a positive reading on efficacy does not just come from random variations but reflects the underlying workings of the compound.
The list of co-signatories is completed by Johnson & Johnson, Merck & Co, Moderna, Novavax and Sanofi.
Read also: Indonesia Eyes Partnership with Bill and Melinda Gates Foundation on Covid-19 Vaccine
Sahin said inclusion in the pledge broadly reflected a significant vaccine market share or a leading position in coronavirus vaccine development.
The frenetic development race has intensified safety concerns about an inoculation, polls have shown.

Western regulators have stressed they would not cut corners but rather prioritize the review workload and allow for development steps in parallel that would normally be handled consecutively.
(Writers: Ludwig Burger, Patricia Weiss | Editor: Susan Fenton)
Simak breaking news dan berita pilihan kami langsung di ponselmu. Pilih saluran andalanmu akses berita Kompas.com WhatsApp Channel : https://www.whatsapp.com/channel/0029VaFPbedBPzjZrk13HO3D. Pastikan kamu sudah install aplikasi WhatsApp ya.