Nearly 2,200 Volunteers Sign Up for Covid-19 Vaccine Trials in Indonesia
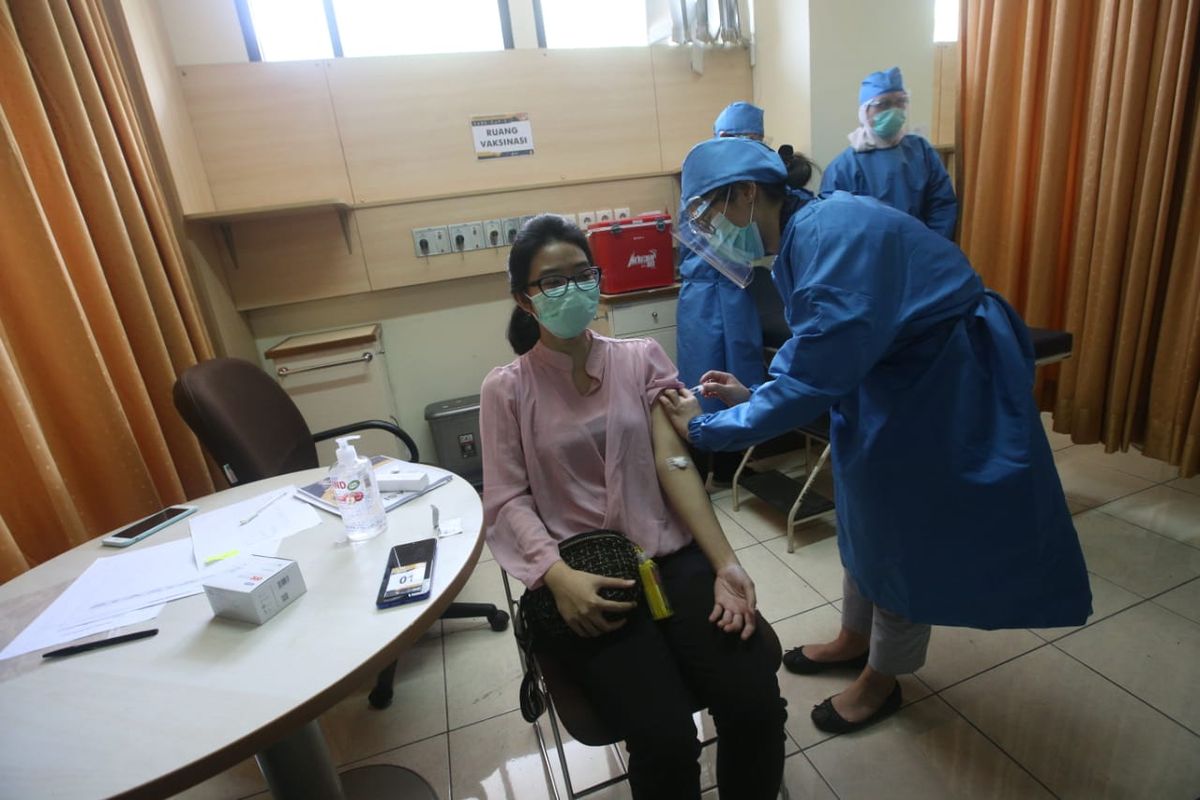
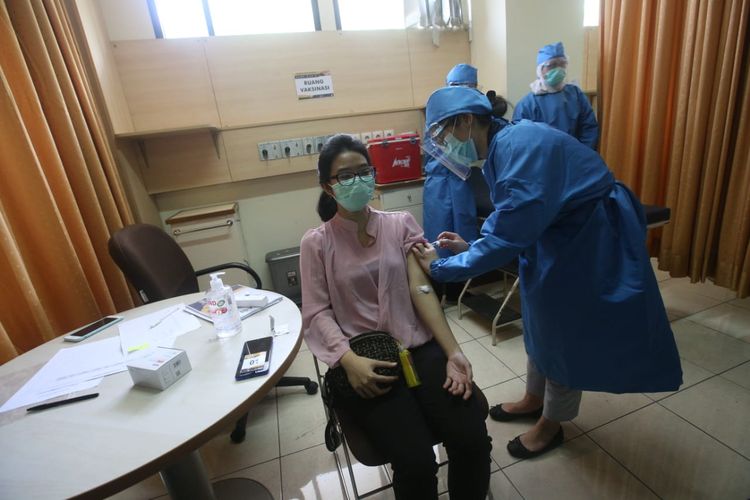
KOMPAS.com – Nearly 2,200 Indonesians have volunteered to roll up their sleeves for Covid-19 vaccine trails, exceeding the initial target of 1,620 as previously announced.
Eddy Fadlyana, field manager of the Covid-19 clinical trials at Padjajaran University’s Faculty of Medicine, said the nearly 2,200 Indonesians are now registered as volunteers.
During the first batch of the injection on August 11-15, 110 volunteers received the shots from the vaccine's clinical trial team in Bandung, West Java.
“It is estimated that the vaccines will be tested on 540 volunteers by November and around 1,620 people by January,” said Eddy.
Not all the volunteers, however, meet the clinical trial requirements.
Also read: Motorcycle Taxi Rider and Family Bravely Roll up Sleeves for Covid-19 Vaccine Trial

“About 10 percent of people failed the screening due to hypertension. They cannot be accepted [into the trial] as volunteers must be in healthy condition,” he said.
After the test was done, Eddy said there were no serious side effects resulting from the injections. “Only one volunteer experienced dizziness, felt unwell. We advise the volunteer to receive treatment at a clinic,” he said.
He went on to say that other volunteers who have already received the shots did not experience side effects. Regarding another volunteer who had a greater appetite after receiving the shot, it was a personal response, he said.
“If the appetite increases it may be a coincidence, it does not happen in general. It is just one person,” he said.
Also read: Indonesia, China Hold Meeting on Covid-19 in Hainan
He explained that the second batch of the test is scheduled to take place on August 28, where 150 volunteers will participate.
The other batches will undergo the tests until next year, while the results of the early batch from the clinical trials are expected to be done in October this year.
“Hopefully this will be completed in January or February,” he said.
The program was part of Phase 3 clinical trials jointly conducted by Indonesian vaccine manufacturer PT Bio Farma and biopharmaceutical company Sinovac Biotech Ltd of China.
PT Bio Farma planned to produce up to 250 million doses of the Covid-19 vaccine early next year after receiving 2,400 candidate vaccines from Sinovac.
(Writer: Jawahir Gustav Rizal | Editor: Jihad Akbar)
Simak breaking news dan berita pilihan kami langsung di ponselmu. Pilih saluran andalanmu akses berita Kompas.com WhatsApp Channel : https://www.whatsapp.com/channel/0029VaFPbedBPzjZrk13HO3D. Pastikan kamu sudah install aplikasi WhatsApp ya.