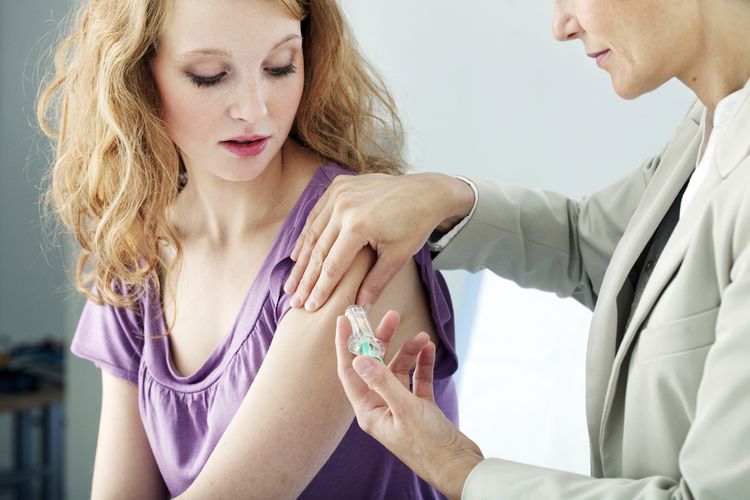
First Covid-19 Vaccine in US Ready for Final Testing
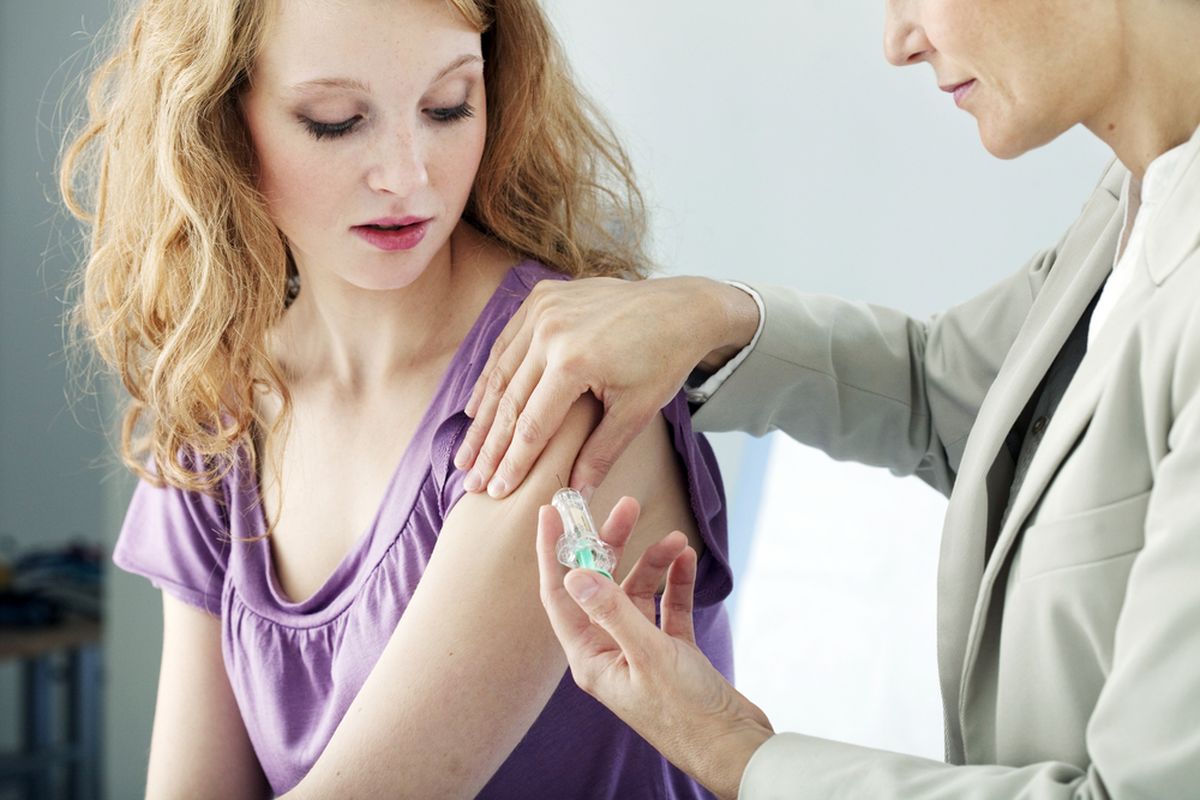
WASHINGTON, KOMPAS.com – The first Covid-19 vaccine tested in the United States has delivered promising signs to ward off the Covid-19 virus.
The vaccine has boosted people’s immune systems the way scientists had hoped, according to researchers.
Researchers anxiously awaited findings from the first 45 volunteers who received a shot of the first Covid-19 vaccine back in March.
The result came back positive as early tests have proven that the first Covid-19 vaccine improved the volunteers’ immune system.
Read also: Indonesia Eyes Local Covid-19 Vaccine Mass Production by Mid-2021
The vaccine is now on its way to begin the crucial round of final testing.
“No matter how you slice this, this is good news,” Dr. Anthony Fauci, the US government’s top infectious disease expert, told AP.
The US’ first Covid-19 vaccine was developed by Dr. Anthony Fauci’s colleagues at the National Institutes of Health and Moderna, Inc.
Final testing is scheduled to take place on July 27 and will involve a 30,000-person study.
The objective is to prove if the first Covid-19 vaccine in the US can sufficiently protect people against the Covid-19 virus.
Read also: Indonesia Seeks Partners for Coronavirus Vaccine Production
Those early volunteers developed what is called neutralizing antibodies in their bloodstream — molecules key to blocking infection — at levels comparable to those found in people who survived Covid-19, the research team reported in the New England Journal of Medicine.
“This is an essential building block that is needed to move forward with the trials that could actually determine whether the vaccine does protect against infection,” said Dr. Lisa Jackson of the Kaiser Permanente Washington Research Institute in Seattle, who led the study.
There’s no guarantee but the government hopes to have results around the end of the year — record-setting speed for developing a vaccine.
The vaccine requires two doses, a month apart.
There were no serious side effects, but more than half the study participants reported flu-like reactions to the shots.
Read also: Don't Expect a Covid-19 Vaccine Anytime Soon: Indonesia’s Erick Thohir
The reactions aren’t uncommon with other vaccines — fatigue, headache, chills, fever, and pain at the injection site.
For three participants given the highest dose, those reactions were more severe; that dose isn’t being pursued.
Some of those reactions are similar to coronavirus symptoms but they’re temporary, lasting about a day and occur right after vaccination, researchers noted.
“Small price to pay for protection against Covid,” said Dr. William Schaffner of Vanderbilt University Medical Center, a vaccine expert who wasn’t involved with the study.
He called the early results “a good first step,” and is optimistic that final testing could deliver answers about whether it's really safe and effective by the beginning of next year.
“It would be wonderful. But that assumes everything’s working right on schedule,” Schaffner cautioned.
Moderna’s share price jumped nearly 15 percent in trading after US markets closed. Shares of the company, based in Cambridge, Massachusetts, have nearly quadrupled this year.
Tuesday's results only included younger adults. The first-step testing later was expanded to include dozens of older adults, the age group most at risk from Covid-19.
Those results aren't public yet but regulators are evaluating them.
Fauci said final testing will include older adults, as well as people with chronic health conditions that make them more vulnerable to the virus — and Black and Latino populations likewise affected.
Nearly two dozen possible Covid-19 vaccines are in various stages of testing around the world.
Candidates from China and Britain’s Oxford University also are entering the final testing stages.
The 30,000-person study will mark the world’s largest study of a potential Covid-19 vaccine so far.
And the NIH-developed shot isn’t the only one set for such massive US testing, crucial to spot rare side effects.
The government plans similar large studies of the Oxford candidate and another by Johnson & Johnson; separately, Pfizer Inc. is planning its own huge study.
Already, people can start signing up to volunteer for different studies.
People think “this is a race for one winner. Me, I’m cheering every one of them on,” said Fauci, who directs NIH’s National Institute of Allergy and Infectious Diseases.
“We need multiple vaccines. We need vaccines for the world, not only for our own country.”
Around the world, governments are investing in stockpiles of hundreds of millions of doses of the different candidates, in hopes of speedily starting inoculations if any are proven to work.
(Writer: Lauran Neergaard, AP Medical Writer)
Source: https://apnews.com/e4d5259bfc6c74fcb090d885737c55a6
Simak breaking news dan berita pilihan kami langsung di ponselmu. Pilih saluran andalanmu akses berita Kompas.com WhatsApp Channel : https://www.whatsapp.com/channel/0029VaFPbedBPzjZrk13HO3D. Pastikan kamu sudah install aplikasi WhatsApp ya.