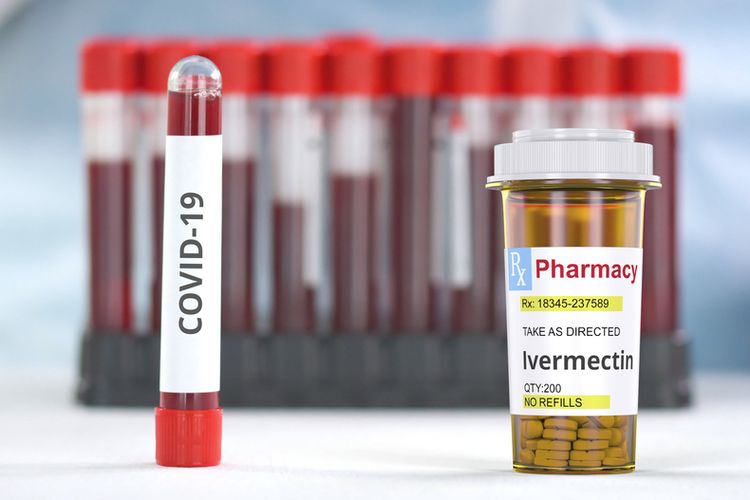
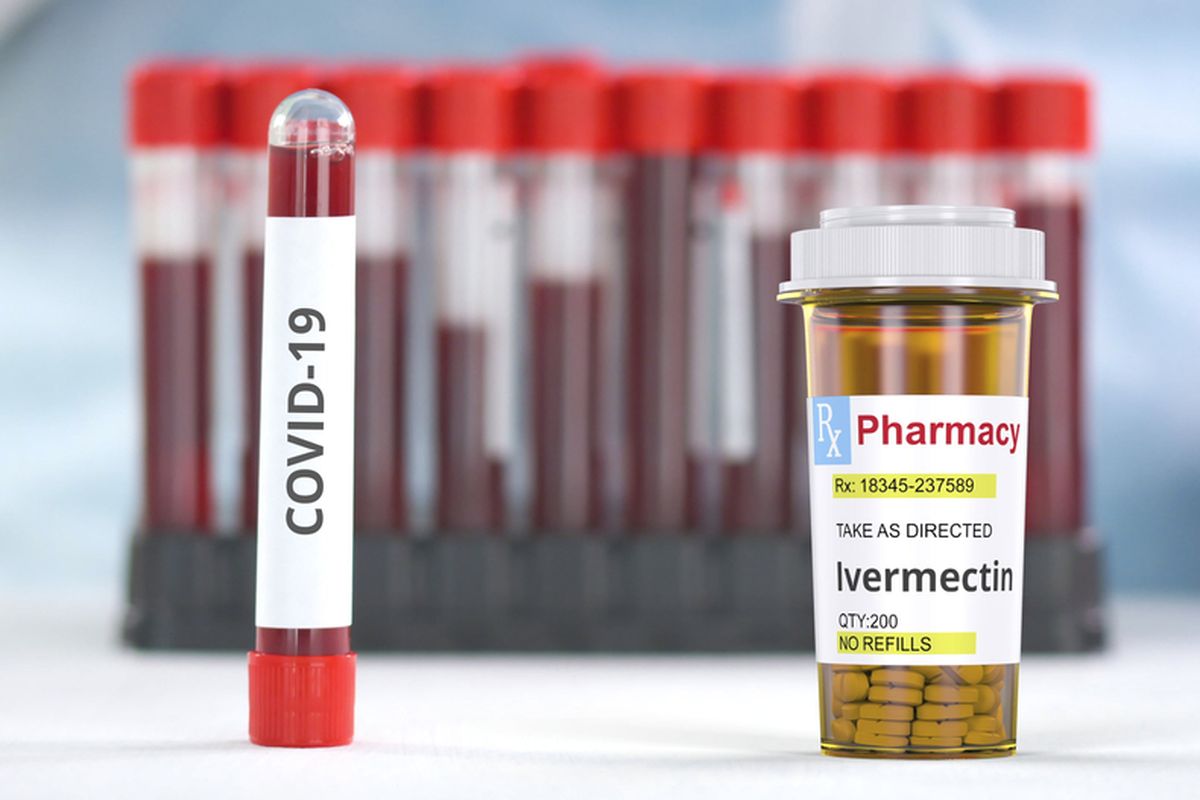
Indonesian Drug Agency Denies Issuing Circular that Regulates Ivermectin Distribution
JAKARTA, KOMPAS.com - Indonesia's Food and Drug Agency (BPOM) head Penny Lukito denied that her office has issued an emergency use authorization (EUA) for Ivermectin to treat Covid-19.
Penny said that clinical trials of Ivermectin, an anti-parasitic medicine for use in treating coronavirus, are still running in eight hospitals.
"No EUA for Ivermectin has been issued as clinical trials have just started," said Penny told Kompas.com on Thursday, July 15.
Also read: Clinical Trials on Ivermectin for Treatment of Covid-19 Infection Approved
Penny said Ivermectin can be obtained in eight hospitals that conduct clinical trials and other hospitals based on technical instructions. Penny urged the public not to take the medicine without a prescription.
Previously, a circular letter that regulates Ivermectin distribution went viral on social media, stating the medicine could be used for treating coronavirus.
Besides Ivermectin, seven other drugs have been authorized by BPOM. This includes Remdesivir, Favipiravir, Oseltamivir, Immunoglobulin, Tocilizumab, Azithromycin, and Dexamethasone.
It was reported that the circular on the Implementation of Drug Distribution with the approval of Emergency Use (Emergency Use Authorization) was distributed by Arya Sinulingga, a special staff member at the State-Owned Enterprises Ministry.
However, at the time of publication, Penny has yet provided detailed information regarding the distribution of the circular that mentioned emergency use authorization for Ivermectin.
(Writer: Akhdi Martin Pratama | Editor: Akhdi Martin Pratama)
Simak breaking news dan berita pilihan kami langsung di ponselmu. Pilih saluran andalanmu akses berita Kompas.com WhatsApp Channel : https://www.whatsapp.com/channel/0029VaFPbedBPzjZrk13HO3D. Pastikan kamu sudah install aplikasi WhatsApp ya.